THANK-uCAR®
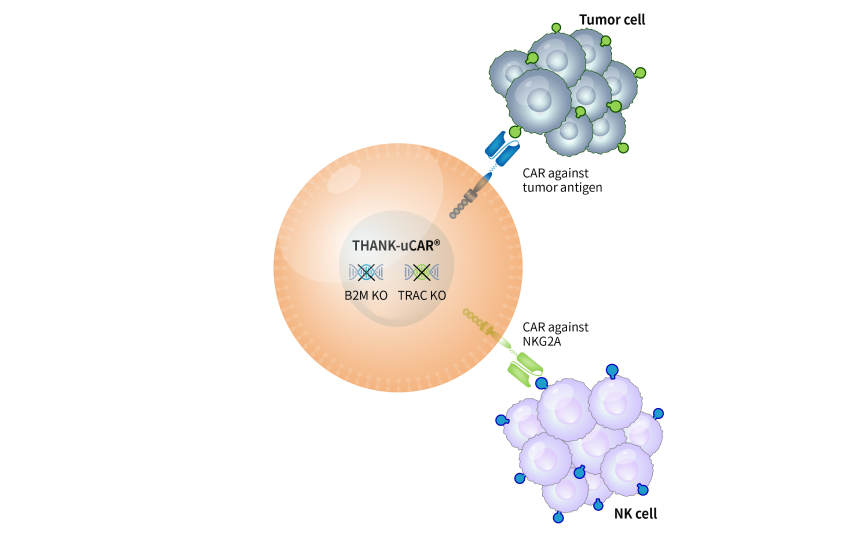
To reduce the rejection of host T cell against allogeneic CAR T cells, B2M gene was generally knockout. However, host NK cells shall attack the allogeneic CAR T cells without B2M, leading to less proliferation and persistence of the allogeneic CAR T cells.
To address this issue, a CAR recognizing NKG2A, a surface protein of NK cells was armored into the allogeneic CAR T cells to hinder the attack of host NK cells, which is so called THANK(Target to Hinder the Attack of NK cells)-uCAR technology. CARsgen’s data demonstrated that THANK-uCAR® T cells could survive and proliferate much better than TCR/B2M double knockout allogeneic T cells in the presence of NK cells.
LADAR®
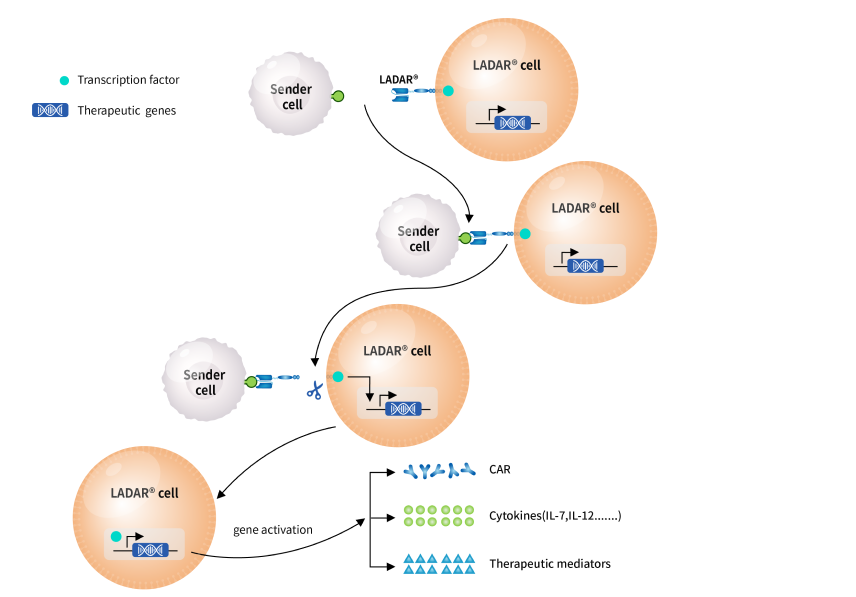
Most tumor-associated antigens are also expressed in normal tissues, which poses great challenges for the development of targeting therapies including immune cell therapies. To resolve the challenge with target availability, CARsgen continues to explore innovative technologies to enhance drug target availability and therefore make undruggable targets druggable. The Company developed LADAR® technology, in which the intracellular transcription of the gene of interest is controlled by an artificial receptor which contains a transcript factor (TF) in the intracellular domain. Once the extracellular domain of the LADAR® receptor binds to a sender antigen, the TF was cleaved and translocated to nucleus to trigger the expression of target genes of interest. The gene of interests could be a chimeric antigen receptor (CAR), a cytokine or any therapeutic mediator you need.
The LADAR-CAR circuits require a sender antigen for LADAR® and a target antigen for CAR recognition to kill target cells and thus reduce on-target off-tumor toxicities if these two antigens are not simultaneously expressed in the same normal tissues. In CARsgen’s in vitro studies, LADAR® system induced strong gene expression in response to sender antigen engagement and importantly, nearly no leakage expression in the absence of sender antigens.
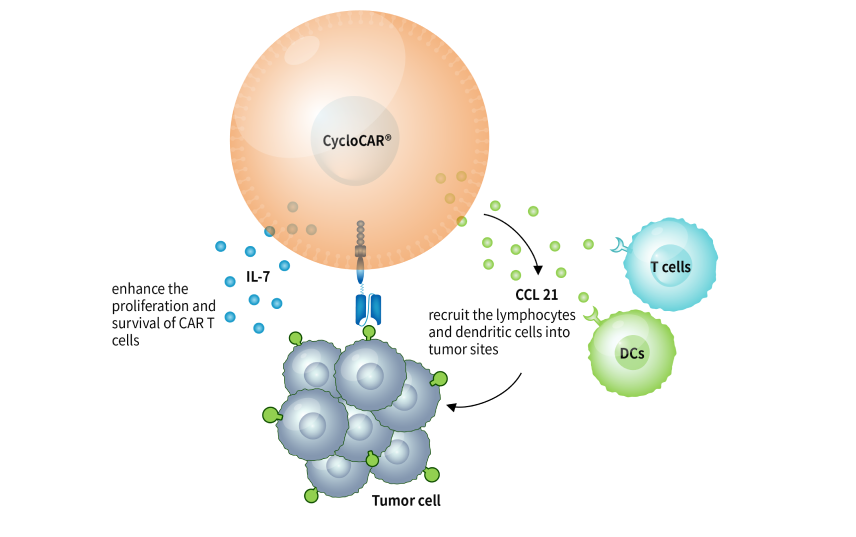
CARsgen is also working on other applications of LADAR® system, such as LADAR-cytokine circuits. The Company believes that LADAR® system is a powerful tool for developing CAR T cells with reduced on-target off-tumor toxicities and expanding the target availability. Additionally, it could also be applied in precise delivery of cytokines and other therapeutic mediators.