About CARsgen®
We focus on innovative CAR T-cell therapies
We are biopharmaceutical company focusing on developing innovative CAR T-cell therapies to address the unmet clinical needs including but not limited to hematologic malignancies, solid tumors and autoimmune diseases.


Pipeline
Innovative and differentiated product pipeline
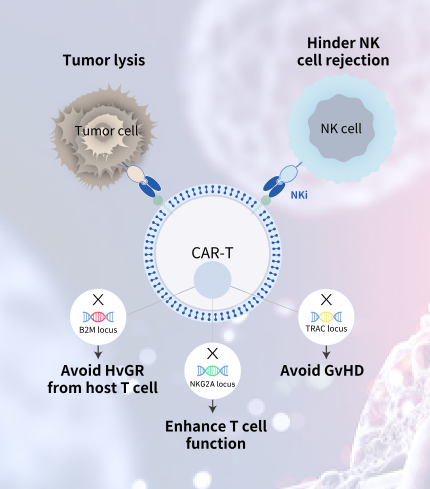
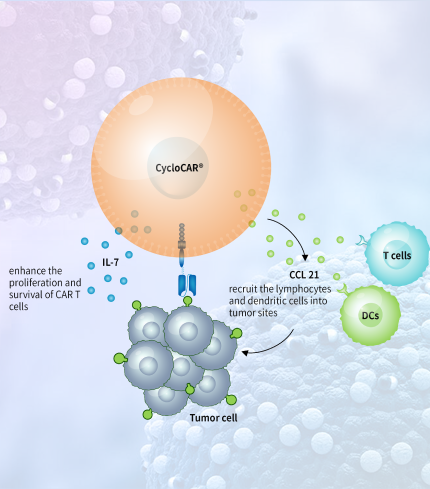
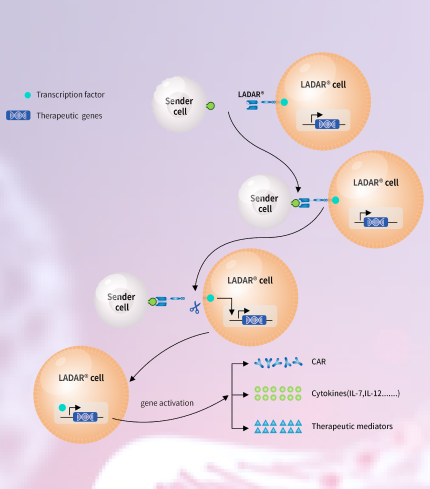
We develop innovative CAR-T technologies to empower the next generation pipeline products.
We have built an integrated cell therapy platform
News Center